DAILY
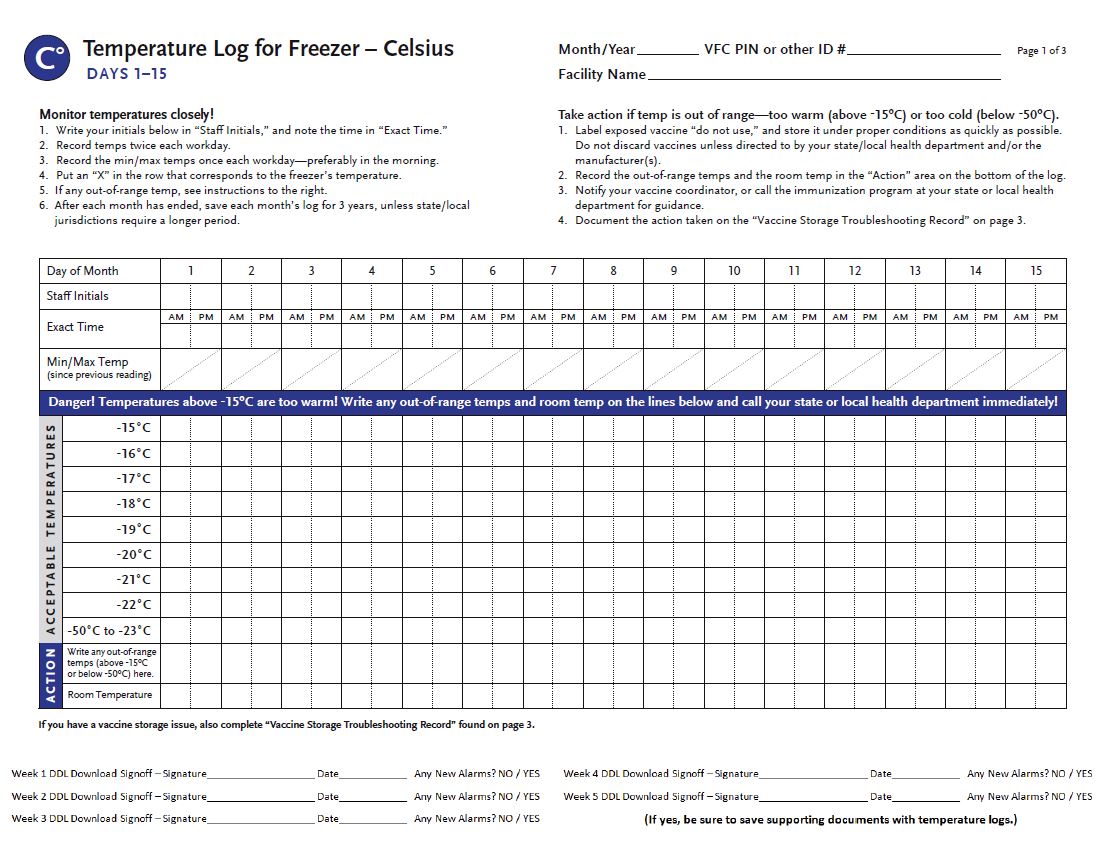
- Temperature monitoring is the primary responsibility of the Primary VFC Contact and/or Back-up Contact. Temperatures are required to be reviewed within each vaccine storage unit twice a day (morning and afternoon 30-60 minutes before the office closes).
- The refrigerator should maintain temperatures between 36°F and 46°F (2°C and 8°C).
- Set the temperature mid-range to achieve an average of 40°F (5°C).
- The freezer should maintain temperatures between -58°F and +5°F (-50°C and -15°C).
- VFC Providers should set a temperature recording interval of one reading every 15 minutes.
- The minimum and maximum temperatures for the last 24 hours must be reviewed and recorded each morning and then reset.
- If your DDL automatically resets at 12 AM, then you will need to manually review the previous 24 hours for min/max temperatures.
- These temperature readings must be documented, as should any actions that are taken if the temperatures readings are out of acceptable range. Save the actual data files, not just the graphs.
- Download and review data from data loggers weekly on Monday morning or the first clinic day after the weekend.
- Write the time and date of each reading, the initials of person who recorded the reading and whether any new alarms were present on each vaccine storage unit’s Digital Data Logger.
- VFC vaccines are clearly labeled and separated from privately purchased vaccine for easy identification.
WEEKLY
The VFC Contact or their Back-up reviews the temperature log for each unit to ensure that the unit was checked twice each clinic day and any new alarms were documented.
- Download each storage unit’ s weekly data logger report from the DDL onto a computer per manufacturer’s instructions on Monday morning or the first clinic day after the weekend.
- Sign & date the bottom of the VFC temperature log to confirm that the weekly report was reviewed. This is only to confirm that the report was reviewed for the week.
- Note or highlight any alarms on the logger report and how staff responded to the alarm. All information about a temperature excursion, including the data logger report and any follow up, should be filed with the temperature logs for the unit and retained for three years.
ADDITIONAL MONITORING ACTIVITIES
- Responsible Medical Providers are accountable for maintaining current and valid certificates of calibration.
- Visually check storage units for correct vaccine and probe placement.
- Inspect the storage units for cleanliness.
- Follow the manufacturer’s maintenance schedule for the storage unit.
- These documents are required to be maintained for three years.
- Install new batteries at least every six months on all data loggers.